Why the Moon Is Rusting: NASA’s Unbelievable Discovery
Moon Rust: Why Is Our Airless Moon Rusting? The Strange Science Explained
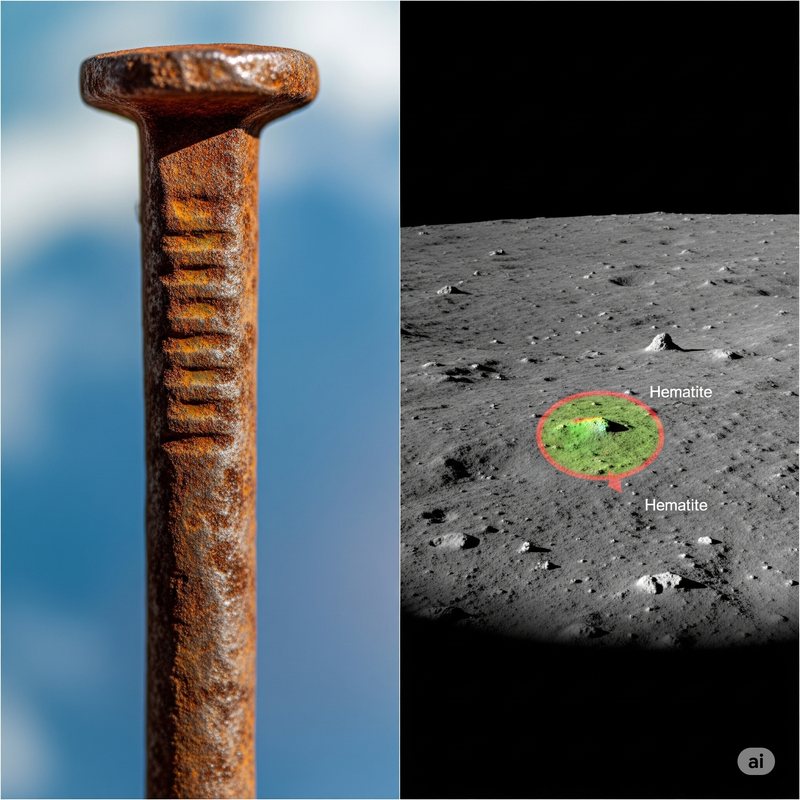
Rust on the Moon? It sounds like something from a sci-fi movie gone wrong. After all, rust, or iron oxide, famously requires two key ingredients: **oxygen and water**. Our Moon, a barren, airless celestial body bombarded by harsh solar winds, is thought to lack both of these in significant amounts. Yet, in a revelation that sent ripples through the planetary science community in 2020, scientists using data from India’s pioneering Chandrayaan-1 mission made a shocking discovery: unmistakable traces of **hematite** (Fe₂O₃), a form of iron oxide, on the lunar surface. This perplexing finding isn't just a quirky anomaly; it profoundly challenges what we thought we knew about lunar geology and opens up exciting new avenues for understanding the intricate dance between Earth and its celestial companion.
What Exactly Is Rust, and Why Is It So Weird on the Moon?
On Earth, we see rust everywhere – on old cars, forgotten tools, and metal structures. It’s the result of a chemical reaction called oxidation, specifically the corrosion of iron when it’s exposed to oxygen and moisture. The basic chemical formula for common rust (hydrated iron(III) oxide) is often simplified as:
$4Fe + 3O₂ + nH₂O → 2Fe₂O₃·nH₂O$
Here’s the catch: the Moon is a vacuum. It has no atmosphere to provide oxygen, no liquid water on its surface, and it’s constantly bombarded by the solar wind – a stream of charged particles (mostly hydrogen ions) from the Sun. These hydrogen ions are highly reductive, meaning they tend to *add* electrons to materials, making it incredibly difficult for oxidation (which involves *losing* electrons) to occur. In fact, solar wind should actively *prevent* rust from forming!
So, the presence of hematite on the Moon isn't just weird; it's a profound scientific paradox that forced researchers to rethink the lunar environment.
The Science Behind Moon Rusting: An Earthly Connection
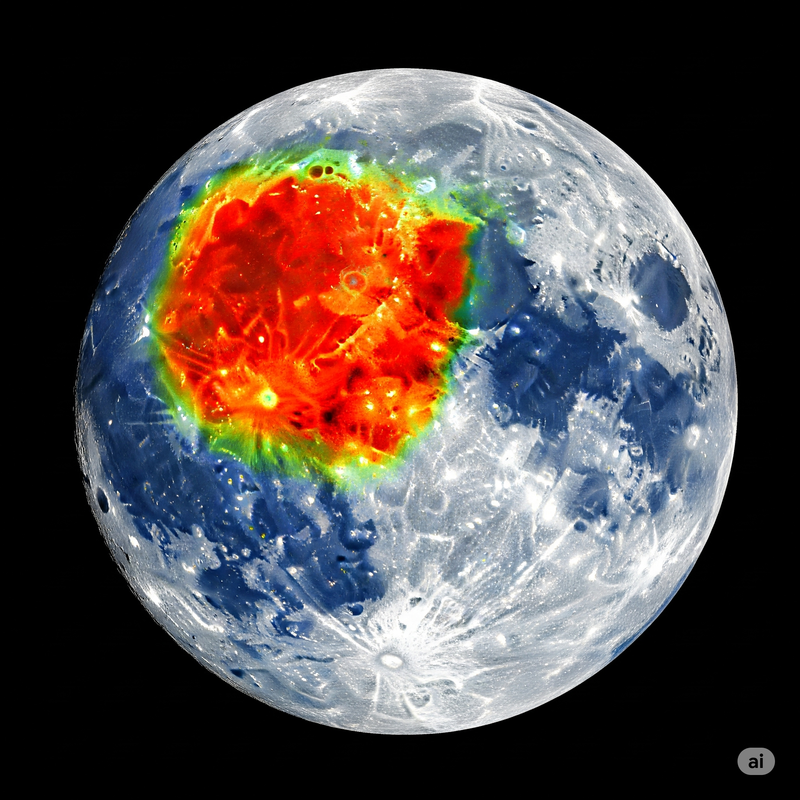
The groundbreaking detection of **hematite** came from data collected by the **Moon Mineralogy Mapper (M3)** instrument aboard India's Chandrayaan-1 orbiter, launched in 2008. Researchers from NASA’s Jet Propulsion Laboratory (JPL) and the University of Hawai‘i meticulously analyzed spectroscopic data, identifying the distinct spectral "fingerprints" of hematite, particularly at higher lunar latitudes and near the poles [1] – regions less exposed to direct solar radiation.
To explain this seemingly impossible phenomenon, scientists have pieced together a compelling hypothesis involving a surprising interaction between Earth and its Moon:
1. Source of Oxygen: Earth's Atmospheric Gift
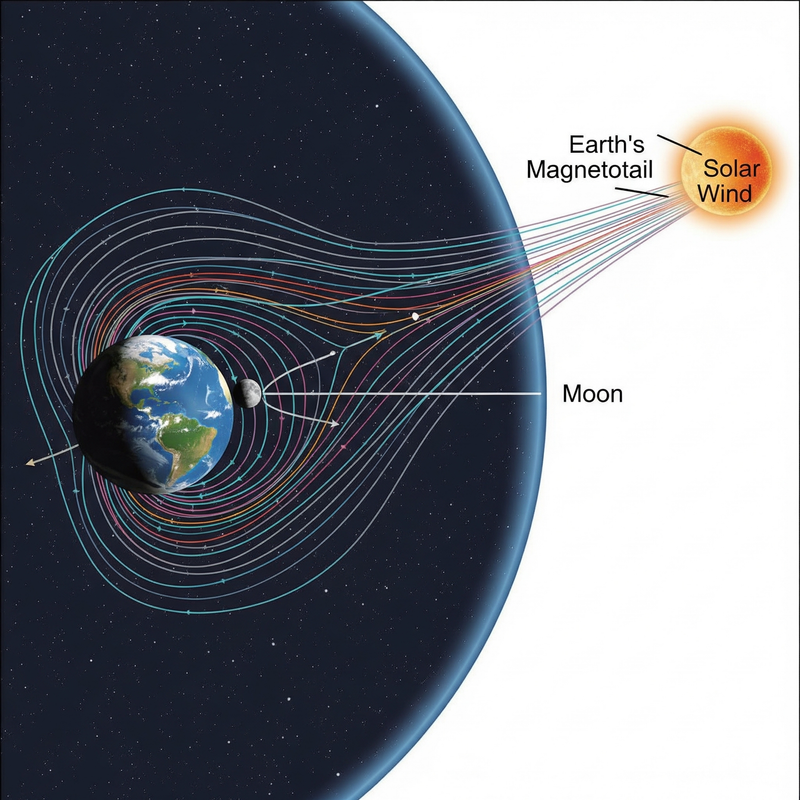
Where could the oxygen possibly come from? The leading theory points to **Earth itself**. Our planet's magnetic field creates a vast protective bubble called the **magnetosphere**, which extends far into space. On the side away from the Sun, this magnetosphere stretches into a long, comet-like structure known as the **magnetotail**, reaching up to 400,000 kilometers (250,000 miles) – far enough to engulf the Moon.
During specific periods of its monthly orbit (roughly five days around the full Moon), the Moon passes through this magnetotail. When this happens, Earth’s upper atmosphere actually leaks trace amounts of **oxygen ions** into space, which are then carried by the magnetotail’s plasma to the lunar surface. While not a strong "wind," this intermittent flow provides just enough oxygen to kickstart the oxidation process [2].
2. Source of Water: Hidden Lunar Hydration
Even with oxygen, rust still needs water. While the Moon is dry, it's not entirely devoid of water. Scientists have found evidence of water ice hidden in permanently shadowed craters at the lunar poles, and there's also ubiquitous evidence of **hydroxyl (OH)** and water molecules adsorbed onto the lunar regolith. These trace amounts of water could come from several sources:
- **Micrometeorite Impacts:** Tiny meteorites hitting the Moon carry small amounts of water, which can be vaporized and then settle into cold traps.
- **Solar Wind Interaction:** While the solar wind primarily carries hydrogen, it can interact with oxygen atoms in lunar dust to form hydroxyl ($OH$) or even water ($H₂O$) molecules in tiny, localized reactions [3].
- **Lunar Interior:** There's also speculation about water trapped deep within the Moon's mantle, slowly outgassing over time.
It's these minute amounts of water, possibly mobilized by impacts or warming during the lunar day, that could react with both iron and Earth-derived oxygen.
3. Shielding from Solar Wind: The Protective Pause
The **solar wind** is a double-edged sword for rust. As mentioned, its hydrogen ions are highly *reductive*, meaning they readily donate electrons, actively preventing iron from losing electrons (oxidizing) and thus stopping rust from forming. However, when the Moon passes through Earth’s magnetotail, it gets a crucial reprieve.
During this period, the Moon is largely **shielded from the constant barrage of solar wind hydrogen** [2]. This temporary "shelter" reduces the reductive environment, allowing the oxygen and trace water to react with the lunar iron, giving hematite a window of opportunity to form. This intermittent shielding is what makes the Earth-Moon connection so critical to the rusting process.
Why This Discovery Is So Important for Science & Space Exploration
The finding of Moon rust isn't just a quirky scientific footnote; it has profound implications across several fields of planetary science:
- **Rethinking Earth-Moon Interactions:** This discovery unequivocally demonstrates that Earth’s magnetic and atmospheric environment extends far enough to have a tangible chemical impact on the Moon. It reveals a more dynamic and interconnected relationship between our planet and its satellite than previously understood, influencing lunar surface chemistry in ways we're just beginning to grasp.
- **Clues About Lunar Water Sources:** The existence of hematite strongly supports the idea that the Moon might harbor more accessible water than previously thought. If water or hydroxyl ions are present and mobile enough to facilitate rusting, it boosts prospects for **in-situ resource utilization (ISRU)**. This means future lunar colonists might be able to extract water for drinking, oxygen for breathing, or hydrogen for rocket fuel directly from lunar materials, reducing the need to transport resources from Earth.
- **Insights into Planetary Evolution:** Studying how rust forms on an airless body like the Moon provides unique insights into oxidation processes that might occur on other celestial objects lacking atmospheres, such as asteroids or the moons of Mars (Phobos and Deimos). It helps scientists refine models of how planetary surfaces evolve over billions of years, especially under various levels of stellar radiation and planetary influence.
- **Revisiting Lunar Geology:** The presence of hematite challenges older assumptions about the Moon's interior. While most iron on the Moon is in a reduced (non-oxidized) state, the existence of hematite suggests that some internal processes might bring oxidized iron to the surface, or that surface conditions are more complex than simple exposure to solar wind.
The Future of Moon Rust Research & Exploration
This rust-colored enigma opens a fascinating new chapter in lunar science. It prompts critical questions for future research:
- Could the Moon’s deep interior be more active than current models suggest, supplying oxidized materials to the surface?
- Are there other unexpected oxidized minerals or elements on the Moon, beyond hematite, waiting to be discovered?
- Can scientists harness this understanding of lunar oxidation to develop new methods for studying lunar water or oxygen transport, perhaps even for generating breathable oxygen in future lunar habitats?
Future missions are eager to dig deeper. NASA’s Artemis program, aiming to return humans to the Moon by 2026, and subsequent robotic missions like India’s Chandrayaan-3 and future lunar landers, are designed to gather direct samples from the lunar poles and other regions. Analyzing these samples on-site or bringing them back to Earth will allow scientists to study the precise composition, age, and formation mechanisms of hematite and other unexpected lunar minerals. These direct analyses will undoubtedly influence how we plan for sustainable human presence and resource utilization on the Moon in the coming decades.
FAQs – Moon Rusting Explained
Got more questions about rust on the Moon? We've got answers!
Q1: How was rust detected on the Moon?
A1: Scientists detected hematite (a form of rust) by analyzing spectral data collected by the **Moon Mineralogy Mapper (M3)** instrument aboard India's Chandrayaan-1 orbiter. M3 identifies minerals by their unique light absorption patterns.
Q2: Is this lunar rust like the rust we see on Earth?
A2: Yes, the detected mineral is **hematite (Fe₂O₃)**, which is the same type of iron oxide found in common terrestrial rust. The conditions for its formation on the Moon, however, are very different and much more unusual.
Q3: Does this mean the Moon has liquid water on its surface?
A3: No, it does not suggest the presence of flowing liquid water on the Moon's surface. Instead, it indicates the presence of trace amounts of water molecules or hydroxyl ions, likely adsorbed onto the lunar regolith or trapped as ice in shadowed regions, which are sufficient for these slow chemical reactions over long periods.
Q4: Can Moon rust be useful for future astronauts or lunar habitats?
A4: Possibly! Understanding the precise conditions and mechanisms of lunar rusting could offer valuable insights into the presence and mobilization of oxygen and water on the Moon. This knowledge is crucial for **in-situ resource utilization (ISRU)**, which aims to extract local resources for oxygen generation, water production, or even construction materials for future lunar bases, reducing reliance on expensive resupply missions from Earth.
Join the Cosmic Conversation!
What do you think causes the Moon to rust? Do you believe Earth influences the Moon more than we realize, or are there other lunar secrets waiting to be uncovered?
Let us know your thoughts in the comments below. If you found this article interesting, share it with fellow space enthusiasts in your circle and help us spread the wonder of science!
Sources and Further Reading:
- JPL, NASA. (2020, September 2). *NASA Finds Moon is Rusting*. https://www.jpl.nasa.gov/news/nasa-finds-moon-is-rusting
- Xu, Y., et al. (2020). *Earth’s Oxygen Influences Moon’s Hematite Formation*. Science Advances, 6(41), eaba1950. https://www.science.org/doi/10.1126/sciadv.aba1950
- Honniball, C. I., et al. (2020). *Molecular water detected on the sunlit Moon by SOFIA*. Nature Astronomy, 4, 1146–1152. (While not directly about rust, this paper confirms widespread molecular water, essential for the rusting process). https://www.nature.com/articles/s41550-020-1198-x
- ISRO. (n.d.). *Chandrayaan-1 Mission*. (Official mission page, for general context). https://www.isro.gov.in/Chandrayaan1.html
Connect with Science by Rao!
Join our community for more fascinating science discussions and updates:
Comments
Post a Comment